Chronic obstructive pulmonary disease
| Chronic Obstructive Pulmonary Disease | |
|---|---|
| Classification and external resources | |
 Gross pathology of a lung showing centrilobular-type emphysema characteristic of smoking. This close-up of the fixed, cut lung surface shows multiple cavities lined by heavy black carbon deposits. |
|
| ICD-10 | J40. - J44., J47. |
| ICD-9 | 490 - 492, 494 - 496 |
| OMIM | 606963 |
| DiseasesDB | 2672 |
| MedlinePlus | 000091 |
| eMedicine | med/373 emerg/99 |
| MeSH | C08.381.495.389 |
Chronic obstructive pulmonary disease (COPD), also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic airflow limitation (CAL) and chronic obstructive respiratory disease (CORD), refers to chronic bronchitis and emphysema, a pair of commonly co-existing diseases of the lungs in which the airways become narrowed.[1] This leads to a limitation of the flow of air to and from the lungs causing shortness of breath. In contrast to asthma, the limitation of airflow is poorly reversible and usually gets progressively worse over time.
COPD is caused by noxious particles or gas, most commonly from tobacco smoking, which triggers an abnormal inflammatory response in the lung.[2][3] The inflammatory response in the larger airways is known as chronic bronchitis, which is diagnosed clinically when people regularly cough up sputum. In the alveoli, the inflammatory response causes destruction of the tissues of the lung, a process known as emphysema. The natural course of COPD is characterized by occasional sudden worsenings of symptoms called acute exacerbations, most of which are caused by infections or air pollution.
The diagnosis of COPD requires lung function tests. Important management strategies are smoking cessation, vaccinations, rehabilitation, and drug therapy (often using inhalers). Some patients go on to require long-term oxygen therapy or lung transplantation.[2]
Worldwide, COPD ranked as the sixth leading cause of death in 1990. It is projected to be the fourth leading cause of death worldwide by 2030 due to an increase in smoking rates and demographic changes in many countries.[4] COPD is the 4th leading cause of death in the U.S., and the economic burden of COPD in the U.S. in 2007 was $42.6 billion in health care costs and lost productivity.[5][6]
Contents |
Classification
The two-fold nature of the pathology has been studied in the past, see [7] for details. Furthermore, also in recent studies, many authors found that each patient could be classified as presenting a predominantly bronchial or emphysematous phenotype simply analyzing clinical, functional, and radiological findings or studying interesting biomarkers.[8][9][10] A statistical model reflecting the specific predominant mechanism of airflow limitation for a specific patient has been developed and trained over a database of hundreds of patients. The model is available here (http://www.clipcopd.com) as a free on-line application.
Chronic bronchitis
Lung damage and inflammation in the large airways results in chronic bronchitis. Chronic bronchitis is defined in clinical terms as a cough with sputum production on most days for 3 months of a year, for 2 consecutive years.[11] In the airways of the lung, the hallmark of chronic bronchitis is an increased number (hyperplasia) and increased size (hypertrophy) of the goblet cells and mucous glands of the airway. As a result, there is more mucus than usual in the airways, contributing to narrowing of the airways and causing a cough with sputum. Microscopically there is infiltration of the airway walls with inflammatory cells. Inflammation is followed by scarring and remodeling that thickens the walls and also results in narrowing of the airways. As chronic bronchitis progresses, there is squamous metaplasia (an abnormal change in the tissue lining the inside of the airway) and fibrosis (further thickening and scarring of the airway wall). The consequence of these changes is a limitation of airflow.[12]
Patients with advanced COPD that have primarily chronic bronchitis rather than emphysema were commonly referred to as "blue bloaters" because of the bluish color of the skin and lips (cyanosis) seen in them.[13] The hypoxia and fluid retention leads to them being called "Blue Bloaters."
Emphysema
Lung damage and inflammation of the air sacs (alveoli) results in emphysema. Emphysema is defined as enlargement of the air spaces distal to the terminal bronchioles, with destruction of their walls.[11] The destruction of air space walls reduces the surface area available for the exchange of oxygen and carbon dioxide during breathing. It also reduces the elasticity of the lung itself, which results in a loss of support for the airways that are embedded in the lung. These airways are more likely to collapse causing further limitation to airflow. The effort made by patients suffering from emphysema during exhalation, causes a pink color in their faces, hence the term commonly used to refer to them, "pink puffers".
Signs and symptoms
Essentials of diagnosis include:
- History of cigarette smoking.
- Chronic cough and sputum production (in chronic bronchitis)
- Dyspnea (in emphysema)
- Rhonchi, decreased intensity of breath sounds, and prolonged expiration on physical examination
- Airflow limitation on pulmonary function testing that is not fully reversible and most often progressive
One of the most common symptoms of COPD is shortness of breath (dyspnea). People with COPD commonly describe this as: "My breathing requires effort," "I feel out of breath," or "I can't get enough air in".[14] People with COPD typically first notice dyspnea during vigorous exercise when the demands on the lungs are greatest. Over the years, dyspnea tends to get gradually worse so that it can occur during milder, everyday activities such as housework. In the advanced stages of COPD, dyspnea can become so bad that it occurs during rest and is constantly present.
Other symptoms of COPD are a persistent cough, sputum or mucus production, wheezing, chest tightness, and tiredness.[15][16]
People with advanced (very severe) COPD sometimes develop respiratory failure. When this happens, cyanosis, a bluish discoloration of the lips caused by a lack of oxygen in the blood, can occur. An excess of carbon dioxide in the blood can cause headaches, drowsiness or twitching (asterixis). A complication of advanced COPD is cor pulmonale, a strain on the heart due to the extra work required by the heart to pump blood through the affected lungs.[17] Symptoms of cor pulmonale are peripheral edema, seen as swelling of the ankles, and dyspnea.
There are a few signs of COPD that a healthcare worker may detect although they can be seen in other diseases. Some people have COPD and have none of these signs. Common signs are:
- tachypnea, a rapid breathing rate
- wheezing sounds or crackles in the lungs heard through a stethoscope
- breathing out taking a longer time than breathing in
- enlargement of the chest, particularly the front-to-back distance (hyperaeration)
- active use of muscles in the neck to help with breathing
- breathing through pursed lips
- increased anteroposterior to lateral ratio of the chest (i.e. barrel chest).
Cause
Smoking
The primary risk factor for COPD is chronic tobacco smoking. In the United States, 80 to 90% of cases of COPD are due to smoking.[18][19] Exposure to cigarette smoke is measured in pack-years[20], the average number of packages of cigarettes smoked daily multiplied by the number of years of smoking. The likelihood of developing COPD increases with age and cumulative smoke exposure, and almost all life-long smokers will develop COPD, provided that smoking-related, extrapulmonary diseases (cardiovascular, diabetes, cancer) do not claim their lives beforehand.[21]
Occupational exposures
Intense and prolonged exposure to workplace dusts found in coal mining, gold mining, and the cotton textile industry and chemicals such as cadmium, isocyanates, and fumes from welding have been implicated in the development of airflow obstruction, even in nonsmokers.[22] Workers who smoke and are exposed to these particles and gases are even more likely to develop COPD. Intense silica dust exposure causes silicosis, a restrictive lung disease distinct from COPD; however, less intense silica dust exposures have been linked to a COPD-like condition.[23] The effect of occupational pollutants on the lungs appears to be substantially less important than the effect of cigarette smoking.[24]
Air pollution
Studies in many countries have found that people who live in large cities have a higher rate of COPD compared to people who live in rural areas.[25] Urban air pollution may be a contributing factor for COPD as it is thought to slow the normal growth of the lungs although the long-term research needed to confirm the link has not been done. In many developing countries indoor air pollution from cooking fire smoke (often using biomass fuels such as wood and animal dung) is a common cause of COPD, especially in women.[26]
Genetics
Some factor in addition to heavy smoke exposure is required for a person to develop COPD. This factor is probably a genetic susceptibility. COPD is more common among relatives of COPD patients who smoke than unrelated smokers.[27] The genetic differences that make some peoples' lungs susceptible to the effects of tobacco smoke are mostly unknown. Alpha 1-antitrypsin deficiency is a genetic condition that is responsible for about 2% of cases of COPD. In this condition, the body does not make enough of a protein, alpha 1-antitrypsin. Alpha 1-antitrypsin protects the lungs from damage caused by protease enzymes, such as elastase and trypsin, that can be released as a result of an inflammatory response to tobacco smoke.[28]
Other risk factors
A tendency to sudden airway constriction in response to inhaled irritants, bronchial hyperresponsiveness, is a characteristic of asthma. Many people with COPD also have this tendency. In COPD, the presence of bronchial hyperresponsiveness predicts a worse course of the disease.[24] It is not known if bronchial hyperresponsiveness is a cause or a consequence of COPD. Other risk factors such as repeated lung infection and possibly a diet high in cured meats may be related to the development of COPD.
Autoimmune disease
There is mounting evidence that there may be an autoimmune component to COPD.[29] Many individuals with COPD who have stopped smoking have active inflammation in the lungs.[30] The disease may continue to get worse for many years after stopping smoking due to this ongoing inflammation.[30] This sustained inflammation is thought to be mediated by autoantibodies and autoreactive T cells.[30][31][32]
Pathophysiology
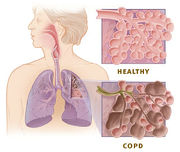
It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are:
- Oxidative stress produced by the high concentrations of free radicals in tobacco smoke.
- Cytokine release due to inflammation as the body responds to irritant particles such as tobacco smoke in the airway.
- Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as alpha 1-antitrypsin, allowing protease enzymes to damage the lung.
Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs. In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways. In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation.[33]
If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started. When this happens, there is an increase in the volume of air in the lungs, a process called dynamic hyperinflation.[33]
Dynamic hyperinflation is closely linked to shortness of breath (dyspnea) in COPD.[34] It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and chest wall when they are already stretched by hyperinflation.
Another factor contributing to shortness of breath in COPD is the loss of the surface area available for the exchange of oxygen and carbon dioxide with emphysema. This reduces the rate of transfer of these gasses between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body. A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation.
Some people with advanced COPD do manage to breathe fast to compensate, but usually have dyspnea as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies but this can eventually lead to headaches, drowsiness and heart failure.
Advanced COPD can lead to complications beyond the lungs such as weight loss (cachexia), pulmonary hypertension and right-sided heart failure (cor pulmonale). Osteoporosis, heart disease, muscle wasting and depression are all more common in people with COPD.[2]
Several molecular signatures associated to lung function decline and corollaries of disease severity have been proposed, a majority of which are characterized in easily accessible surrogate tissue, including blood derivatives such as serum and plasma. A recent 2010 clinical study proposes alpha 1B-glycoprotein precursor/A1BG, Alpha 2-antiplasmin, apolipoprotein A-IV precursor/APOA4, and complement component 3 precursor, among other coagulation and complement system proteins as corrollaries of lung function decline, although ambiguity between cause and effect is unresolved.[35]
Acute exacerbations of COPD
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. It may be triggered by an infection with bacteria or viruses or by environmental pollutants. Typically, infections cause 75% or more of the exacerbations; bacteria can roughly be found in 25% of cases, viruses in another 25%, and both viruses and bacteria in another 25%. Pulmonary Embolism can also cause exacerbations of COPD. Airway inflammation is increased during the exacerbation resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer. This can also lead to hypo ventilation and eventually hypoxia, thus can lead to insufficient tissue perfusion then cell necrosis.[2]
Diagnosis
The diagnosis of COPD should be considered in anyone who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease such as regular tobacco smoking.[2][36] No single symptom or sign can adequately confirm or exclude the diagnosis of COPD[37] although COPD is uncommon under the age of 40 years.
Spirometry
The diagnosis of COPD is confirmed by spirometry,[2] a test that measures breathing. Spirometry measures the forced expiratory volume in one second (FEV1) which is the greatest volume of air that can be breathed out in the first second of a large breath. Spirometry also measures the forced vital capacity (FVC) which is the greatest volume of air that can be breathed out in a whole large breath. Normally at least 70% of the FVC comes out in the first second (i.e. the FEV1/FVC ratio is >70%). In COPD, this ratio is less than normal, (i.e. FEV1/FVC ratio is <70%) even after a bronchodilator medication has been given.
Spirometry can help to determine the severity of COPD.[2] The FEV1 (measured post-bronchodilator) is expressed as a percent of a predicted "normal" value based on a person's age, gender, height and weight:
| Severity of COPD | FEV1 % predicted |
|---|---|
| Mild | ≥80 |
| Moderate | 50–79 |
| Severe | 30–49 |
| Very severe | <30 or Chronic respiratory failure symptoms |
The severity of COPD also depends on the severity of dyspnea and exercise limitation. These and other factors can be combined with spirometry results to obtain a COPD severity score that takes multiple dimensions of the disease into account.[38]
Other tests
An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema. A high-resolution computed tomography scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases.
A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polycythemia), a reaction to long-term hypoxemia.
Management
There is currently no cure for COPD; however, COPD is both a preventable and treatable disease. Clinical practice guidelines for the management of COPD are available from the Global Initiative for Chronic Obstructive Lung Disease (GOLD),[39] a collaboration that includes the World Health Organization and the U.S. National Heart, Lung, and Blood Institute. The major current directions of COPD management are to assess and monitor the disease, reduce the risk factors, manage stable COPD, prevent and treat acute exacerbations and manage comorbidity.[2]
The only measures that have been shown to reduce mortality is smoking cessation and supplemental oxygen.[40]
Risk factor reduction
Smoking cessation
Smoking cessation is one of the most important factors in slowing down the progression of COPD. Once COPD has been diagnosed, stopping smoking slows down the rate of progression of the disease. Even at a late stage of the disease it can significantly reduce the rate of deterioration in lung function and delay the onset of disability and death.[12] It is the only standard intervention that can improve the rate of progression of COPD.[40]
Smoking cessation starts with an individual decision to stop smoking that leads to an attempt at quitting. Often several attempts are required before long-term smoking cessation is achieved.[41] Some smokers can achieve long-term smoking cessation through "willpower" alone. However smoking is highly addictive[42] and many smokers need further support to quit. The chance of successfully stopping smoking can be greatly improved through social support, engagement in a smoking cessation programme and the use of drugs such as nicotine replacement therapy, bupropion and varenicline.[41]
The policies of governments, public health agencies and anti-smoking organizations can reduce smoking rates by encouraging smoking cessation and discouraging people from starting smoking.[41] These policies are important strategies in the prevention of COPD.
Occupational health
Measures can be taken to reduce the likelihood that workers in at-risk industries such as coal mining will develop COPD. Some examples of these measures are: education of workers and management about the risks, promoting smoking cessation, surveillance of workers for early signs of COPD, the use of personal dust monitors, the use of respirators and dust control.[43] Dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation. If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role.
Air pollution
Air quality can be improved by pollution reduction efforts which should lead to health gains for people with COPD. A person who has COPD may experience fewer symptoms if they stay indoors on days when air quality is poor.[2]
Bronchodilators
Bronchodilators are medicines that relax smooth muscle around the airways, increasing the calibre of the airways and improving air flow. They can reduce the symptoms of shortness of breath, wheeze and exercise limitation, resulting in an improved quality of life for people with COPD.[44] They do not slow down the rate of progression of the underlying disease.[2] Bronchodilators are usually administered with an inhaler or via a nebulizer.
There are two major types of bronchodilator, β2 agonists and anticholinergics. Anticholinergics appear to be superior to β2 agonists in COPD. Anticholinergics reduce respiratory deaths while β2 agonists have no effect on respiratory deaths.[45] Each type may be either long-acting (with an effect lasting 12 hours or more) or short-acting (with a rapid onset of effect that does not last as long).
β2 agonists
β2 agonists stimulate β2 receptors on airway smooth muscles, causing them to relax. There are several β2 agonists available. Albuterol (common brand name: Ventolin) and terbutaline are widely used short acting β2 agonists and provide rapid relief of COPD symptoms. Long acting β2 agonists (LABAs) such as salmeterol and formoterol are used as maintenance therapy and lead to improved airflow, exercise capacity, and quality of life.[46]
Anticholinergics
Anticholinergic drugs cause airway smooth muscles to relax by blocking stimulation from cholinergic nerves. Ipratropium is the most widely prescribed short acting anticholinergic drug. Like short-acting β2 agonists, short-acting anticholinergics provide rapid relief of COPD symptoms and a combination of the two is commonly used for a greater bronchodilator effect. Tiotropium is the most commonly prescribed long-acting anticholinergic drug in COPD. It has more specificity for M3 muscarinic receptors so may have fewer side-effects than other anticholinergic drugs. Regular use is associated with improvements in airflow, exercise capacity, quality of life and possibly a longer life. [47][48] In January 2010, new research showed that ipratropium used to treat COPD increased cardiovascular morbidity.[49] At the same time Tiotropium was shown to be effective in eliminating the risk of all cause mortality, cardiovascular mortality and cardiovascular events.[50]
Corticosteroids
Corticosteroids act to reduce the inflammation in the airways, in theory reducing lung damage and airway narrowing caused by inflammation.[51] Unlike bronchodilators, they do not act directly on the airway smooth muscle and do not provide immediate relief of symptoms. Some of the more common corticosteroids in use are prednisone, fluticasone, budesonide, mometasone, and beclomethasone. Corticosteroids are used in tablet or inhaled form to treat and prevent acute exacerbations of COPD. Well-inhaled corticosteroids (ICS) have not been shown to be of benefit for people with mild COPD, however, they have been shown to decrease acute exacerbations in those with either moderate or severe COPD.[52] They however have no effect on overall one-year mortality and are associated with increased rates of pneumonia.[40]
Other medication
Theophylline is a bronchodilator and phosphodiesterase inhibitor that in high doses can reduce symptoms for some people who have COPD. More often, side effects such as nausea and stimulation of the heart limit its use.[2] In lower doses, it may slightly reduce the number of COPD exacerbations.[53] The investigative phosphodiesterase-4 antagonists, roflumilast and cilomilast have completed Phase-2 clinical trials. Tumor necrosis factor antagonists such as infliximab suppress the immune system and reduce inflammation. Infliximab has been trialled in COPD but there was no evidence of benefit with the possibility of harm.[54]
Supplemental oxygen

Supplemental oxygen can be given to people with COPD who have low oxygen levels in the body. Oxygen is provided from an oxygen cylinder or an oxygen concentrator and delivered to a person through tubing via a nasal cannula or oxygen mask. Supplemental oxygen does not greatly improve shortness of breath but can allow people with COPD and low oxygen levels to do more exercise and household activity. Long-term oxygen therapy for at least 16 hours a day can improve the quality of life and survival for people with COPD and arterial hypoxemia or with complications of hypoxemia such as pulmonary hypertension, cor pulmonale, or secondary erythrocytosis.[55] High concentrations of supplemental oxygen can lead to the accumulation of carbon dioxide and respiratory acidosis for some people with severe COPD; lower oxygen flow rates are generally safer for these individuals.
Pulmonary rehabilitation
Pulmonary rehabilitation is a program of exercise, disease management and counselling coordinated to benefit the individual.[56] Pulmonary rehabilitation has been shown to improve shortness of breath and exercise capacity. It has also been shown to improve the sense of control a patient has over their disease as well as their emotions.[57]
Nutrition
Being either underweight or overweight can affect the symptoms, degree of disability and prognosis of COPD. People with COPD who are underweight can improve their breathing muscle strength by increasing their calorie intake.[2] When combined with regular exercise or a pulmonary rehabilitation programme, this can lead to improvements in COPD symptoms.
Surgery
Surgery is sometimes helpful for COPD in selected cases. A bullectomy is the surgical removal of a bulla, a large air-filled space that can squash the surrounding, more normal lung. Lung volume reduction surgery is similar; parts of the lung that are particularly damaged by emphysema are removed allowing the remaining, relatively good lung to expand and work better. Lung transplantation is sometimes performed for severe COPD, particularly in younger individuals.
Prognosis
COPD usually gradually gets worse over time and can lead to death. The rate at which it gets worse varies between individuals. The factors that predict a poorer prognosis are:[2]
- Severe airflow obstruction (low FEV1)
- Poor exercise capacity
- Shortness of breath
- Significantly underweight or overweight
- Complications like respiratory failure or cor pulmonale
- Continued smoking
- Frequent acute exacerbations
Epidemiology

In England, an estimated 842,100 of 50 million people have a diagnosis of COPD; translating into approximately one person in 59 receiving a diagnosis of COPD at some point in their lives. In the most socioeconomically deprived parts of the country, one in 32 people were diagnosed with COPD, compared with one in 98 in the most affluent areas. [59] In the United States, the prevalence of COPD is approximately 1 in 20 or 5%, totalling approximately 13.5 million people in USA,[60] or possibly approximately 25 million people if undiagnosed cases are included.[61]
History
COPD has probably always existed but has been called by different names in the past. Bonet described a condition of “voluminous lungs” in 1679. In 1769, Giovanni Morgagni described 19 cases where the lungs were “turgid” particularly from air.[62] The first description and illustration of the enlarged airspaces in emphysema was provided by Ruysh in 1721."History of pathologic descriptions of COPD" (PDF). http://www.mhprofessional.com/downloads/products/0071457399/0071457399_chap40.pdf. Matthew Baillie illustrated an emphysematous lung in 1789 and described the destructive character of the condition.[62] Badham used the word "catarrh" to describe the cough and mucus hypersecretion of chronic bronchitis in 1814. He recognised that chronic bronchitis was a disabling disorder.
René Laennec, the physician who invented the stethoscope, used the term "emphysema" in his book A Treatise on the Diseases of the Chest and of Mediate Auscultation (1837) to describe lungs that did not collapse when he opened the chest during an autopsy. He noted that they did not collapse as usual because they were full of air and the airways were filled with mucus.[62]
In 1842, John Hutchinson invented the spirometer, which allowed the measurement of vital capacity of the lungs. However, his spirometer could only measure volume, not airflow.[63] Tiffeneau in 1947 and Gaensler in 1950 and 1951 described the principles of measuring airflow.
The terms chronic bronchitis and emphysema were formally defined at the CIBA guest symposium of physicians in 1959. The term COPD was first used by William Briscoe in 1965 and has gradually overtaken other terms to become established today as the preferred name for this disease.
See also
- COPD Awareness Month
- Restrictive lung disease
- Obstructive lung disease
- Hypoxic drive
Footnotes
- ↑ U.S. National Heart Lung and Blood Institute - What is COPD
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Rabe KF, Hurd S, Anzueto A, et al. (2007). "Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary". Am. J. Respir. Crit. Care Med. 176 (6): 532–55. doi:10.1164/rccm.200703-456SO. PMID 17507545.
- ↑ Hogg JC, Chu F, Utokaparch S, et al. (2004). "The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease". N. Engl. J. Med. 350 (26): 2645–53. doi:10.1056/NEJMoa032158. PMID 15215480.
- ↑ Mathers CD, Loncar D (November 2006). "Projections of global mortality and burden of disease from 2002 to 2030". PLoS Med. 3 (11): e442. doi:10.1371/journal.pmed.0030442. PMID 17132052. PMC 1664601. http://dx.plos.org/10.1371/journal.pmed.0030442.
- ↑ COPD (Chronic Obstructive Pulmonary Disease)
- ↑ "2007 NHLBI Morbidity and Mortality Chart Book" (PDF). http://www.nhlbi.nih.gov/resources/docs/07a-chtbk.pdf. Retrieved 2008-06-06.
- ↑ Burrows B, Fletcher CM, Heard BE, et al (1966). "The emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and Chicago". Lancet 87: 830–5.
- ↑ Kitaguchi Y, Fujimoto K, Kubo K, Honda T (October 2006). "Characteristics of COPD phenotypes classified according to the findings of HRCT". Respir Med 100 (10): 1742–52. doi:10.1016/j.rmed.2006.02.003. PMID 16549342. http://linkinghub.elsevier.com/retrieve/pii/S0954-6111(06)00064-3.
- ↑ Paoletti M, Camiciottoli G, Meoni E, et al. (December 2009). "Explorative data analysis techniques and unsupervised clustering methods to support clinical assessment of Chronic Obstructive Pulmonary Disease (COPD) phenotypes". J Biomed Inform 42 (6): 1013–21. doi:10.1016/j.jbi.2009.05.008. PMID 19501190. http://linkinghub.elsevier.com/retrieve/pii/S1532-0464(09)00080-X.
- ↑ Petty TL (2002). "COPD: clinical phenotypes". Pulm Pharmacol Ther 15 (4): 341–51. doi:10.1006/pupt.2002.0380. PMID 12220938. http://linkinghub.elsevier.com/retrieve/pii/S1094-5539(02)90380-9.
- ↑ 11.0 11.1 Longmore, J. M.; Murray Longmore; Wilkinson, Ian; Supraj R. Rajagopalan (2004). Oxford handbook of clinical medicine. Oxford [Oxfordshire]: Oxford University Press. pp. 188–9. ISBN 0-19-852558-3.
- ↑ 12.0 12.1 Kumar P, Clark M (2005). Clinical Medicine (6th ed.). Elsevier Saunders. pp. 900–1. ISBN 0702027634.
- ↑ Chung C, Delaney J, Hodgins R (2008). "Respirology". In Somogyi, Ron; Colman, Rebecca. The Toronto notes 2008: a comprehensive medical reference and review for the Medical Council of Canada Qualifying Exam - Part 1 and the United States Medical Licensing Exam - Step 2. Toronto: Toronto Notes for Medical Students. p. R9. ISBN 0-9685928-8-0.
- ↑ Mahler DA (2006). "Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease". Proceedings of the American Thoracic Society 3 (3): 234–8. doi:10.1513/pats.200509-103SF. PMID 16636091.
- ↑ U.S. National Heart Lung and Blood Institute - Signs and Symptoms
- ↑ MedlinePlus Encyclopedia Chronic obstructive pulmonary disease
- ↑ MedicineNet.com - COPD signs & symptoms
- ↑ MedicineNet.com - COPD causes
- ↑ Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD (August 2009). "COPD prevalence is increased in lung cancer, independent of age, sex and smoking history". Eur. Respir. J. 34 (2): 380–6. doi:10.1183/09031936.00144208. PMID 19196816.
- ↑ "Definition of pack year - NCI Dictionary of Cancer Terms". http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=306510.
- ↑ doi:10.1016/S0140-6736(06)68516-4
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Devereux, Graham (May 2006). "ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors". BMJ 332 (7550): 1142–4. doi:10.1136/bmj.332.7550.1142. PMID 16690673.
- ↑ Hnizdo E, Vallyathan V (April 2003). "Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence". Occup Environ Med 60 (4): 237–43. doi:10.1136/oem.60.4.237. PMID 12660371.
- ↑ 24.0 24.1 Loscalzo, Joseph; Fauci, Anthony S.; Braunwald, Eugene; Dennis L. Kasper; Hauser, Stephen L; Longo, Dan L. (2008). Harrison's Principles of Internal Medicine (17th ed.). McGraw-Hill Professional. ISBN 0-07-146633-9.
- ↑ Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM (September 2006). "Global burden of COPD: systematic review and meta-analysis". Eur. Respir. J. 28 (3): 523–32. doi:10.1183/09031936.06.00124605. PMID 16611654.
- ↑ Kennedy SM, Chambers R, Du W, Dimich-Ward H (December 2007). "Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men?". Proceedings of the American Thoracic Society 4 (8): 692–4. doi:10.1513/pats.200707-094SD. PMID 18073405. http://pats.atsjournals.org/cgi/content/full/4/8/692.
- ↑ Silverman EK, Chapman HA, Drazen JM, et al. (June 1998). "Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis". Am. J. Respir. Crit. Care Med. 157 (6 Pt 1): 1770–8. PMID 9620904. http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=9620904.
- ↑ MedlinePlus Encyclopedia 000091
- ↑ Agustí A, MacNee W, Donaldson K, Cosio M. (2003). "Hypothesis: does COPD have an autoimmune component?". Thorax 58 (10): 832–4. doi:10.1136/thorax.58.10.832. PMID 14514931.
- ↑ 30.0 30.1 30.2 Rutgers, Steven R.; Postma, Dirkje S.; Ten Hacken, Nick H. .T.; Kauffman, Henk F.;van der Mark,Thomas W; Koeter, Gerard H.; Timens, Wim (2000). "Ongoing airway inflammation in patients with COPD who do not currently smoke". Thorax 55 (1): 12–18. doi:10.1136/thorax.55.1.12. PMID 10607796.
- ↑ Feghali-Bostwick CA, Gadgil AS, Otterbein LE, et al. (January 2008). "Autoantibodies in patients with chronic obstructive pulmonary disease". Am. J. Respir. Crit. Care Med. 177 (2): 156–63. doi:10.1164/rccm.200701-014OC. PMID 17975205.
- ↑ Lee SH, Goswami S, Grudo A, et al. (May 2007). "Antielastin autoimmunity in tobacco smoking-induced emphysema". Nat. Med. 13 (5): 567–9. doi:10.1038/nm1583. PMID 17450149.
- ↑ 33.0 33.1 Calverley PM, Koulouris NG (2005). "Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology". Eur Respir J 25 (1): 186–199. doi:10.1183/09031936.04.00113204. PMID 15640341.
- ↑ O'Donnell DE (2006). "Hyperinflation, Dyspnea, and Exercise Intolerance in Chronic Obstructive Pulmonary Disease". The Proceedings of the American Thoracic Society 3 (2): 180–4. doi:10.1513/pats.200508-093DO. PMID 16565429.
- ↑ Rana GS, York TP, Edmiston JS, Zedler BK, et al. (2010). "Proteomic biomarkers in plasma that differentiate rapid and slow decline in lung function in adult cigarette smokers with chronic obstructive pulmonary disease (COPD)". Anal Bioanal Chem 397 (5): 1809–19. doi:10.1007/s00216-010-3742-4. PMID 20442989.
- ↑ Badgett RG, Tanaka DJ, Hunt DK, et al. (1994). "The clinical evaluation for diagnosing obstructive airways disease in high-risk patients". Chest 106 (5): 1427–31. doi:10.1378/chest.106.1427 (inactive 2010-04-06). PMID 7956395.
- ↑ Holleman DR, Simel DL (1995). "Does the clinical examination predict airflow limitation?". JAMA 273 (4): 313–9. doi:10.1001/jama.273.4.313 (inactive 2010-04-29). PMID 7815660.
- ↑ Celli BR, Cote CG, Marin JM, et al. (March 2004). "The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease". N. Engl. J. Med. 350 (10): 1005–12. doi:10.1056/NEJMoa021322. PMID 14999112.
- ↑ Global Initiative for Chronic Obstructive Lung Disease.
- ↑ 40.0 40.1 40.2 Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E (November 2008). "Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis". JAMA 300 (20): 2407–16. doi:10.1001/jama.2008.717. PMID 19033591.
- ↑ 41.0 41.1 41.2 "World Health Organization Tobacco Free Initiative - Policy recommendations for smoking cessation and treatment of tobacco dependence". http://www.who.int/tobacco/resources/publications/tobacco_dependence/en/. Retrieved 28 July 2008.
- ↑ "Why is smoking addictive?". NHS Choices. http://www.nhs.uk/chq/Pages/2278.aspx?CategoryID=53&SubCategoryID=536.
- ↑ Handbook for Dust Control in Mining. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2003-147, Information Circular 9465, 2003 Jun; :1-131. Accessed March 26, 2009.
- ↑ Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koëter GH, Postma DS, Kerstjens HA (February 2002). "A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD". Chest 121 (2): 597–608. doi:10.1378/chest.121.2.597. PMID 11834677. http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=11834677.
- ↑ Salpeter SR, Buckley NS, Salpeter EE (October 2006). "Meta-analysis: anticholinergics, but not beta-agonists, reduce severe exacerbations and respiratory mortality in COPD". J Gen Intern Med 21 (10): 1011–9. doi:10.1111/j.1525-1497.2006.00507.x. PMID 16970553.
- ↑ Calverley PM, Anderson JA, Celli B, et al. (2007). "Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease". N. Engl. J. Med. 356 (8): 775–89. doi:10.1056/NEJMoa063070. PMID 17314337.
- ↑ Tashkin DP, Celli B, Senn S, et al. (October 2008). "A 4-year trial of tiotropium in chronic obstructive pulmonary disease". N. Engl. J. Med. 359 (15): 1543–54. doi:10.1056/NEJMoa0805800. PMID 18836213.
- ↑ O'Donnell DE, Flüge T, Gerken F, et al. (June 2004). "Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD". Eur Respir J 23 (6): 832–40. doi:10.1183/09031936.04.00116004. PMID 15218994. http://erj.ersjournals.com/cgi/pmidlookup?view=long&pmid=15218994.
- ↑ Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD (January 2010). "Cardiovascular events associated with ipratropium bromide in COPD". Chest 137 (1): 13–9. doi:10.1378/chest.08-2367. PMID 19363211. http://chestjournal.chestpubs.org/content/137/1/13.abstract.
- ↑ Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP (January 2010). "Cardiovascular safety of tiotropium in patients with COPD". Chest 137 (1): 20–30. doi:10.1378/chest.09-0011. PMID 19592475. http://chestjournal.chestpubs.org/content/137/1/20.abstract.
- ↑ Pinto-Plata VM, Müllerova H, Toso JF, et al. (January 2006). "C-reactive protein in patients with COPD, control smokers and non-smokers". Thorax 61 (1): 23–8. doi:10.1136/thx.2005.042200. PMID 16143583.
- ↑ Gartlehner G, Hansen RA, Carson SS, Lohr KN (2006). "Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes". Ann Fam Med 4 (3): 253–62. doi:10.1370/afm.517. PMID 16735528.
- ↑ Zhou Y, Wang X, Zeng X, et al. (2006). "Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year". Respirology 11 (5): 603–10. doi:10.1111/j.1440-1843.2006.00897.x. PMID 16916334.
- ↑ Rennard SI, Fogarty C, Kelsen S, et al. (May 2007). "The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease". Am. J. Respir. Crit. Care Med. 175 (9): 926–34. doi:10.1164/rccm.200607-995OC. PMID 17290043.
- ↑ "Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party". Lancet 1 (8222): 681–6. March 1981. PMID 6110912. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(81)91970-X.
- ↑ U.S. National Heart Lung and Blood Institute - Treatment
- ↑ Lacasse Y, Brosseau L, Milne S, et al. (2002). "Pulmonary rehabilitation for chronic obstructive pulmonary disease". Cochrane database of systematic reviews (Online) (3): CD003793. doi:10.1002/14651858.CD003793. PMID 12137716.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html. Retrieved Nov. 11, 2009.
- ↑ Simpson CR, Hippisley-Cox J, Sheikh A (2010). "Trends in the epidemiology of chronic obstructive pulmonary disease in England: a national study of 51804 patients". Brit J Gen Pract 60 (576): 483–488. doi:10.3399/bjgp10X514729.
- ↑ wrongdiagnosis.com > Prevalence and Incidence of COPD Retrieved on Mars 14, 2010
- ↑ MORBIDITY & MORTALITY: 2009 CHART BOOK ON CARDIOVASCULAR, LUNG, AND BLOOD DISEASES National Heart, Lung, and Blood Institute
- ↑ 62.0 62.1 62.2 Petty TL (2006). "The history of COPD". Int J Chron Obstruct Pulmon Dis 1 (1): 3–14. PMID 18046898.
- ↑ Fishman AP (May 2005). "One hundred years of chronic obstructive pulmonary disease". Am. J. Respir. Crit. Care Med. 171 (9): 941–8. doi:10.1164/rccm.200412-1685OE. PMID 15849329.
External links
- COPD-BOLD International Research Platform
- National Heart, Lung and Blood Institute - COPD U.S. NHLBI Information for Patients and the Public page.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)
- "Economic Impact of COPD and Cost Effective Solutions". Access Economics. The Australian Lung Foundation. October 2008. http://www.accesseconomics.com.au/publicationsreports/showreport.php?id=178.
- About COPD Medical information about COPD for patients and their families.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||